

Juri Möbus, Quentin Bonnin, Kirika Ueda, Roland Fröhlich, Kenichiro Itami, Gerald Kehr, and Gerhard Erker
Angew. Chem. Int. Ed. Early View. DOI: 10.1002/anie.201107398
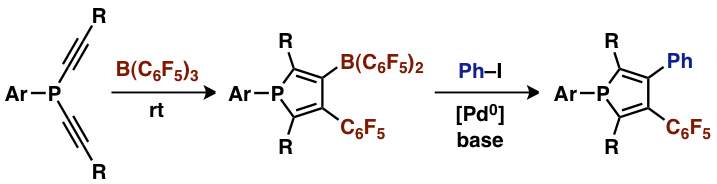
B(C6F5)3 efficiently con- verts a series of bis(alkynyl)phosphines into highly substituted 3-borylphospholes through a twofold 1,1-carboboration reaction sequence. The boron substituted phospholes were also used as substrates in Suzuki–Miyaura type cross-coupling reactions (see scheme).