

Takehisa Maekawa, Yasutomo Segawa, and Kenichiro Itami
Chem. Sci. 2013, Accepted Manuscript. DOI: 10.1039/C3SC50585E
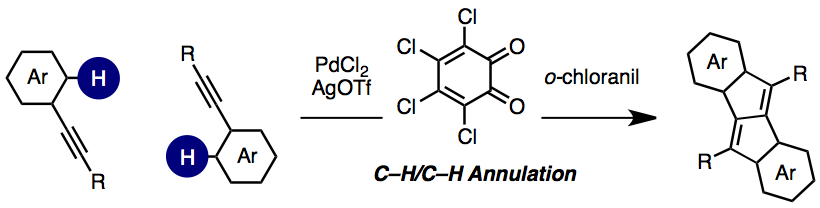
A novel catalytic C-H activation route to privileged dibenzo[a,e]pentalene (DBP) structures has been established. In the presence of PdCl2, AgOTf, and o-chloranil, a C-H/C-H annulation of arylacetylenes takes place to furnish the corresponding DBPs. A number of mechanistic experiments indicate that this new annulation occurs through alkyne-directed, ortho-selective, electrophilic aromatic C-H palladation. Not only symmetric diarylacetylenes but also unsymmetric arylacetylenes are applicable to this reaction. UV-vis absorption spectra and DFT studies on the resulting DBPs indicate a strong substituent effect on the energy levels of HOMO and HOMO-1 of DBPs.