

Kenta Kato, Yasutomo Segawa, and Kenichiro Itami
Can. J. Chem. 2016, Just-in article, DOI: 10.1139/cjc-2016-0467
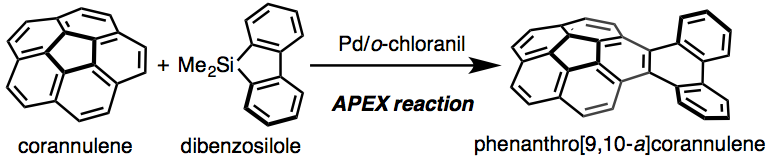
The one-step π-extension of corannulene was achieved using a palladium-catalyzed C-H coupling reaction. The X-ray crystal structure and photophysical properties of the thus formed phenanthro[9,10-a]corannulene (1) were investigated, and the structural properties of 1 were examined by density functional theory calculations. In contrast to dibenzo[g,p]chrysene, the most stable structure of 1 was a butterfly-shaped structure, resulting from the bowl-shaped distortion of the corannulene moiety.