

Keika Hattori, Kazuya Yamaguchi, Junichiro Yamaguchi and Kenichiro Itami
Tetrahedron, 2012, in press. DOI: 10.1016/j.tet.2012.05.091
Special Issue in honor of Professor Manfred D. Reetz. Reetz for receipt of the Tetrahedron Prize 2011
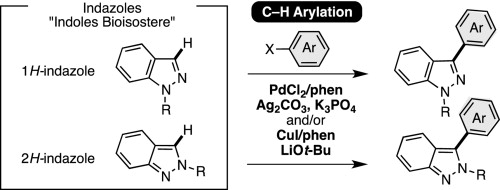
The palladium- and copper-catalyzed C–H arylation reactions of 1H- and 2H-indazoles with haloarenes are described. A PdCl2/phen/Ag2CO3/K3PO4 catalytic system is effective for the C–H arylation of 1H- and 2H-indazoles with haloarenes, whereas a less expensive CuI/phen/LiOt-Bu catalytic system is applicable to the C–H coupling of substituted 2H-indazoles and iodoarenes. The utility of newly developed catalyst was demonstrated in the rapid synthesis of YC-1 (an antitumor agent) and YD-3 (platelet anti-aggregating agent). These new reactions represent important direct functionalization tools of indazoles, well-known bioisosteres of pharmaceuitically important indole core.