

Takao Fujikawa, Yasutomo Segawa, and Kenichiro Itami
J. Org. Chem. 2017, ASAP. DOI: 10.1021/acs.joc.7b01540
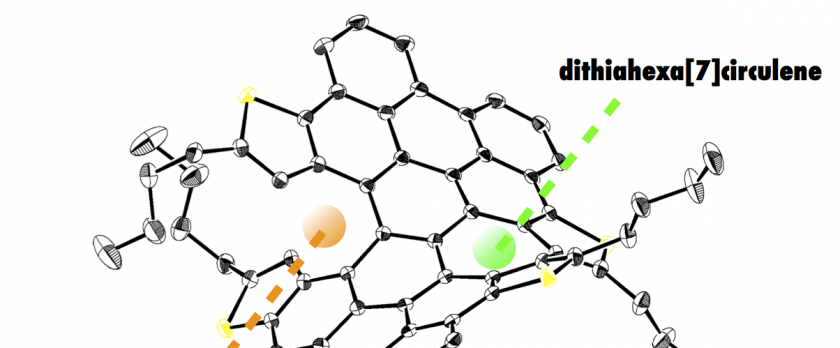
A laterally π-extended dithia[6]helicene, representing an interesting saddle-helix hybrid molecule containing an unusual heptagon, has been synthesized by MoCl5-mediated oxidative stitching of tetrakis(thienylphenyl)naphthalene precursor involving reactive-site capping by chlorination, and subsequent Pd-mediated dechlorination of a tetrachlorinated intermediate. Highly distorted, wide helical structures of π-extended dithia[6]helicenes were clarified by single crystal X-ray diffraction analyses, where heterochiral slipped π-π stacking was displayed in one-dimensional fashion. Notably, theoretical studies on the thermodynamic behavior of π-extended dithia[6]helicene predicted an extraordinary high isomerization barrier of 49.7 kcal·mol-1, which enabled optical resolution and chiroptical measurements. Electronic structures of these huge helicenes were also examined by photophysical and electrochemical measurements.