

Mari Shibata, Hideto Ito,* Kenichiro Itami*
Chem. Lett. 2017, 46, 1701–1704. DOI: 10.1246/cl.170723.
Dedicated issue to the late Prof. Yoshihiko Ito.
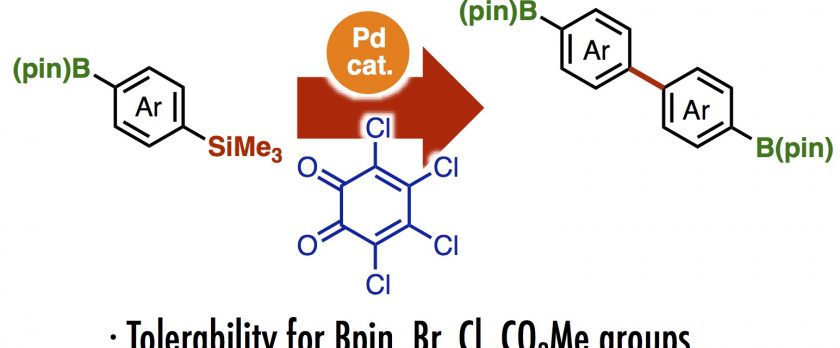
A practical oxidative homocoupling reaction of aryltrimethylsilanes has been achieved by Pd/o-chloranil catalytic system. The reaction shows the good functional group tolerability toward bromo, fluoro, ester and methoxy groups to give a series of biaryls bearing electron-withdrawing and donating groups. The boronate group is also retained on biaryls without any Ar–B bond cleavage, which is highly advantageous for orthogonal coupling.