Takehisa Maekawa, Hiromi Sekizawa, and Kenichiro Itami Angew. Chem. Int. Ed. 2011, in press. DOI: 10.1002/anie.201102092
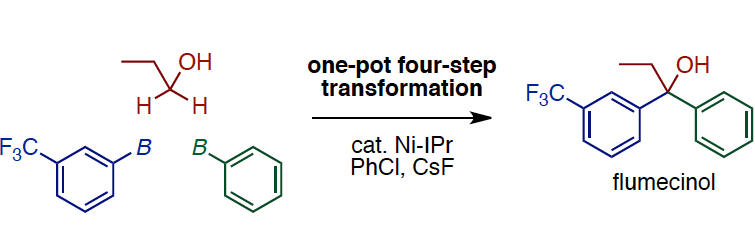
All in one pot: A general synthetic plat- form allows the interconversion of alco- hols and carbonyl compounds in a pre- dictable and controlled fashion in one pot. Under the action of a Ni catalyst, PhCl, CsF, and arylboronates, several
multistep alcohol–carbonyl interconver- sions have been achieved with good overall efficiency (see scheme). A one-pot Ni-catalyzed synthesis of flumecinol (a hepatic microsomal enzyme inducer) has also been demonstrated.