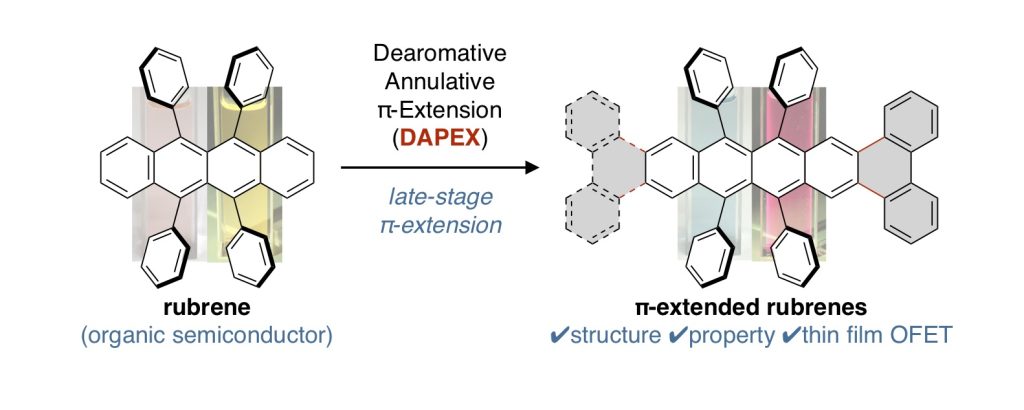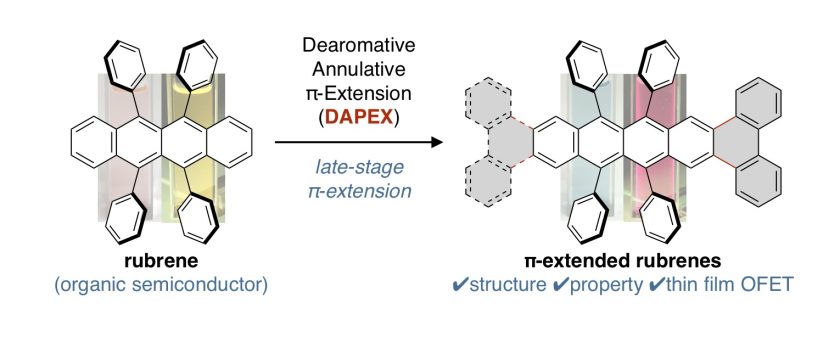
π-Extended Rubrenes via Dearomative Annulative π-Extension Reaction
Wataru Matsuoka, Kou P. Kawahara, Hideto Ito*, David Sarlah, and Kenichiro Itami*
J. Am. Chem. Soc. 2023, 145, 658–666. DOI: 10.1021/jacs.2c11338
ルブレンは有機電子材料への応用研究が古くから知られている芳香族炭化水素であり、関連するアセン類の合成や物性研究が広く進められている。今回我々は、反応後期段階修飾が困難であると考えられてきたルブレンを原料に、これまで開発してきた脱芳香族的縮環π拡張反応(DAPEX)を用いることで、あらたらπ拡張ルブレン類を合成することに成功した。合成したπ拡張ルブレン類は、ルブレンと同様な電子構造を有するが、アセン方向へのπ拡張によって吸収帯・発光帯が大きく長波長シフトした。X線結晶構造解析やなどにより、電荷移動に有利なslipped brick-wall型の結晶配置をとることがわかった。また、ホール輸送性能を調べるためにOFETデバイスを作成したところ、ルブレンと同程度の正孔輸送能をもつことがわかった。
本研究は、アメリカ、イリノイ大学David Sarlah教授との共同研究であり、以前のDAPEX反応の研究(Nat. Commun. 2021, 12, 3940 )に引き続き、2報目の論文です!!おめでとう!
また、本研究は東京化成工業株式会社(TCI)の有機トランジスタ性能評価サービスを利用してOFETデバイス評価を行いました(詳細はこちら:https://www.tcichemicals.com/JP/ja/product/organic-electronics/organic-transistor/featured-articles/index)