

Christina Meyer, Benedikt Neue, Dirk Schepmann, Shuichi Yanagisawa, Junichiro Yamaguchi, Ernst-Ulrich Würthwein, Kenichiro Itami, Bernhard Wünsch
Org. Biomol. Chem. 2011, Advanced Article DOI: 10.1039/C1OB06149F
Late-Stage Arylation:
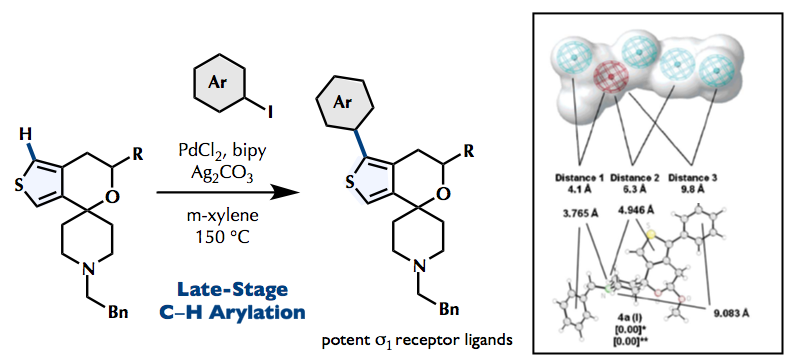
The hypothesis that the s1 receptor will tolerate an additional aryl moiety in position 1 of the spirocyclic system was based on spirocyclic pyrazole derivatives, pharmacophore models of s1 receptor ligands and DFT calculations. The strategy of introducing the aryl residue at the final step of the synthesis allowed the preparation of a large set of diverse ligands for the exploitation of the hydrophobic pocket of the sigma 1 receptor protein. The catalyst system PdCl2/2,2¢-bipyridyl/Ag2CO3 is able to introduce various aryl groups onto the a-positions of spirocyclic thiophene derivatives to afford the target aryl-appended spirocyclic thiophenes . Although the s1 affinity of the 1-phenyl substituted spirocyclic thiophenes is slightly reduced compared with the s1 affinity ofthenon-arylatedcompounds5and6,bothcompoundsrepresentverypotents1 receptorligands. This result indicates that an aryl moiety in position is well tolerated by the sigma 1 receptor protein. The substitution pattern of the additional phenyl moiety has only weak effects onthes1 affinity.Evenligands withextendednaphthylresidueshowhigh sigma1 affinity.However, decreaseofs1 affinitybyextensionofthep-systemtoabiphenylylsubstituent indicates thatthebiphenylylresidueistoolargefortheprimaryhydrophobicbindingpocketofthes1 receptor.