

Katsuma Matsui, Masako Fushimi, Yasutomo Segawa, and Kenichiro Itami
Org. Lett. 2016, ASAP. DOI: 10.1021/acs.orglett.6b02702
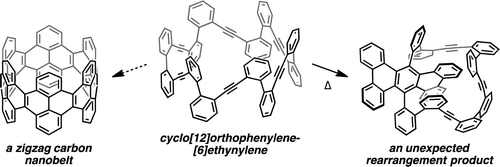
Benzannulated cyclacenes (BCs) have been proposed as stable zigzag carbon nanobelts. Density functional theory (DFT) calculations revealed a closed-shell ground state for [12]BC, whereas an open-shell ground state was suggested for [12]cyclacene. The calculated strain energy and frontier molecular orbital energies of [12]BC also implied high stability. An unstrained macrocycle 1, consisting of orthophenylene and ethynylene moieties, was designed as a potential precursor for [12]BC and synthesized by sequential Suzuki–Miyaura cross-coupling of diphenylacetylene derivatives. While the conversion of 1 into [12]BC is still under investigation, an unexpected rearrangement of the triene moieties in 1, affording a tribenzo[f,k,m]tetraphene structure, was discovered during the screening of reaction conditions. An attempt was made to rationalize this result by proposing a plausible reaction mechanism that proceeds via intermediates containing cyclobutadiene or Dewar benzene moieties. The proposed mechanism is partially supported by DFT calculations.