

Yoshito Koga, Takeshi Kaneda, Yutaro Saito, Kei Murakami,* Kenichiro Itami*
Science 2018, 359, 435–439. DOI: 10.1126/science.aap9801
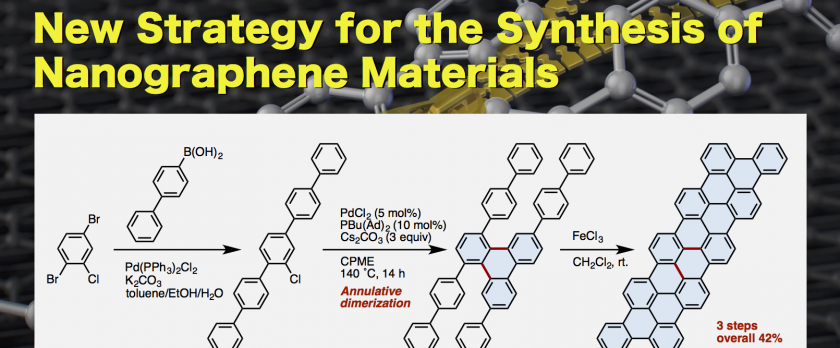
Since the discovery by Ullmann and Bielecki in 1901, reductive dimerization (or homocoupling) of aryl halides has been extensively exploited for the generation of a range of biaryl-based functional molecules. In contrast to the single-point connection in these products, edge-sharing fused aromatic systems have not generally been accessible from simple aryl halides via annulation cascades. Here we report a single-step synthesis of fused aromatics with a triphenylene core by the palladium-catalyzed annulative dimerization of structurally and functionally diverse chlorophenylenes through double carbon-hydrogen bond activation. The partially fused polyaromatics can be transformed into fully fused, small graphene nanoribbons, which are otherwise difficult to synthesize. This simple, yet powerful, method allows access to functional π-systems of interest in optoelectronics research.
Highlighted in …
Chunichi Shimbun, NHK, NIKKAN KOGYO SHIMBUN, Asahi Shimbun