

Marc Steinmetz, Kirika Ueda, Stefan Grimme, Junichiro Yamaguchi, Sylvia Kirchberg, Kenichiro Itami, and Armido Studer
Chem. Asian J. 2012, Early View. DOI: 10.1002/asia.201101011
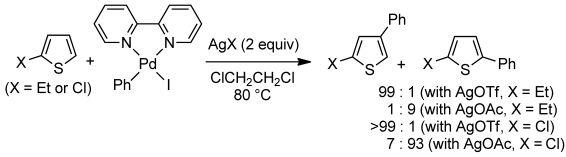
Direct C–H phenylation of 2-ethylthiophene and 2-chlorothiophene with PhPdI(bipy) complex to form either the corresponding 4-phenyl or 5-phenylthiophene derivative is studied under stoichiometric conditions using various Lewis acids as additives. It is shown that reactions occur via the corresponding cationic Pd complex (PhPdbipy+) and that the counteranion determines the regioselectivity. High-level DFT calculations reveal that C–C bond formation occurs via a carbopalladation pathway and not via electrophilic palladation. These calculations give some indications regarding the regioselectivity of the thiophene arylation.