

Takao Fujikawa , Yasutomo Segawa , and Kenichiro Itami
J. Am. Chem. Soc., 2015, DOI: 10.1021/jacs.5b03118
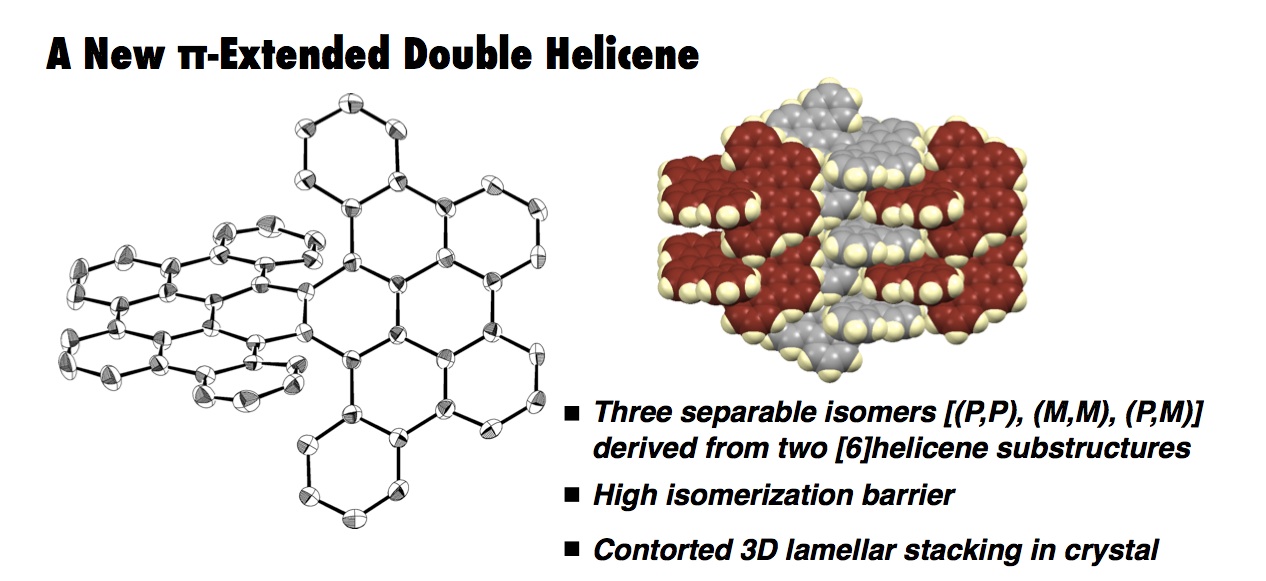
The synthesis, structures, and properties of a π-extended double helicene 1 are described. This double helicene 1 was synthesized by a four-fold oxidative C–H biphenylation of naphthalene followed by the Scholl reaction or via five steps including the Suzuki–Miyaura cross-coupling reaction and the Scholl reaction. Due to the two helical substructures, 1 has three isomers, i.e., two enantiomers in a twisted form [(P,P) and (M,M)] and one diastereoisomer in a meso form. X-ray crystallographic analysis of the twisted isomers (twisted-1) revealed a tightly offset packing pattern of (P,P)- and (M,M)-twisted isomers, affording a three-dimensional lamellar stacking structure. A high isomerization barrier (43.5 kcal mol–1) and the relative thermal stability of twisted-1 isomer over meso-1 by 0.9 kcal mol–1 were estimated by DFT calculations. The three isomers were successfully separated by chiral HPLC and characterized by circular dichroism spectroscopy as well as by TD-DFT studies. Electronic state variation resulting from the molecular geometry difference between the two diastereoisomers (twisted-1 and meso-1) was observed by UV–vis absorption and fluorescence spectra.