

Takao Fujikawa, Yasutomo Segawa, and Kenichiro Itami
J. Am. Chem. Soc. 2016, ASAP. DOI: 10.1021/jacs.6b01303
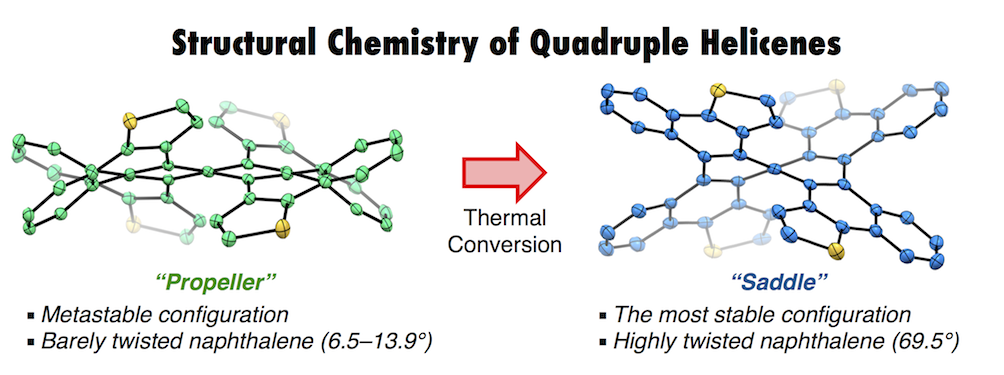
Quadruple helicenes, bearing dithia[6]helicene and [5]helicene substructures, were prepared by a well-controlled Scholl reaction. The four-fold helicity provides nine stereoisomers including four pairs of enantiomers and one meso-isomer. Among them, differently distorted structures of a propeller-shaped isomer (QH-A) and a saddle-shaped isomer (QH-B) were unambiguously determined by X-ray crystallography. Especially in the latter isomer, a proper accumulation of repulsions on the helical substructures twisted the naphthalene core to the limit (69.5°), the highest degree of twisting deformation per benzene unit (35.3° at the most). Photophysical and electrochemical studies showed a broadened HOMO-LUMO gap and a lower lying HOMO of QH-B compared to those of QH-A. These results together with the DFT calculation have clearly demonstrated the electronic state dependency on the molecular geometry. Additionally, kinetic studies of the isomerization between these isomers using 1H NMR, circular dichroism, and DFT calculations shed light on the interconversion pathways among the stereoisomers. The height of barriers in the inversion of a certain helical substructure may be affected by the neighboring helical substructures.