

Hiroki Kondo, Kenichiro Itami, Junichiro Yamaguchi
Chem Sci. 2017, Advance Article. DOI: 10.1039/C7SC00071E
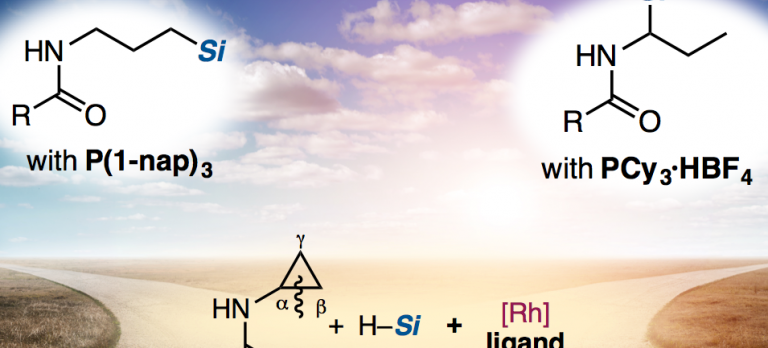
A Rh-catalyzed regiodivergent hydrosilylation of acyl aminocyclopropanes has been developed. Acyl aminocyclopropanes were reacted with hydrosilanes in the presence of Rh catalysts to afford ring-opened hydrosilylated adducts through carbon–carbon (C–C) bond cleavage of the cyclopropane ring. The regioselectivity of the addition of silanes (linear or branched) can be switched by changing the monophosphine ligand. This C–C bond cleavage/hydrosilylation methodology is applicable to the synthesis of silanediol precursors.
Dr. Hiroki Kondo with his accepted article. After acceptance of this paper, he received his PhD degree and graduated. Congrats!!