

Asako, T.; Hayashi, W.; Suzuki, S.; Amaike, K.; Itami, K.; Muto, K.; Yamaguchi, J.
Tetrahedron 2017, accepted (Invited contribution). DOI: 10.1016/j.tet.2017.03.095
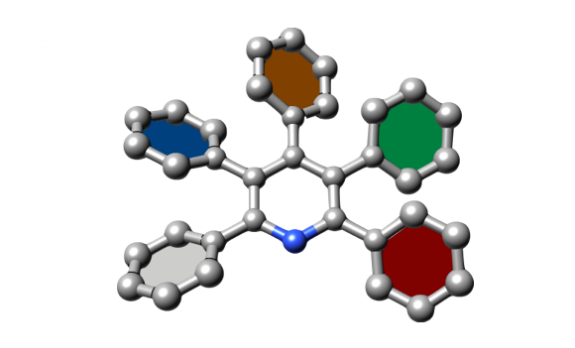
We have achieved a synthesis of multiply arylated pyridines by using a [4+2] cycloaddition of 2,4-diaryl-5-chloroxazoles and cinnamic acids as a key reaction. The resulting hydroxytriarylpyridines can be derivatized into triarylpyridines, tetraarylpyridines and pentaarylpyridines by sequential cross-couplings. This synthetic method allows for facile and rapid access to highly arylated pyridines with different aryl substituents.
Mr. Suzuki (left) and Ms. Hayashi (right)

Mr. Asako in Yamaguchi group in Waseda University. Congrats!