

Mari Shibata, Hideto Ito,* Kenichiro Itami*
J. Am. Chem. Soc. 2018, 140, 2196–2205. DOI: 10.1021/jacs.7b11260
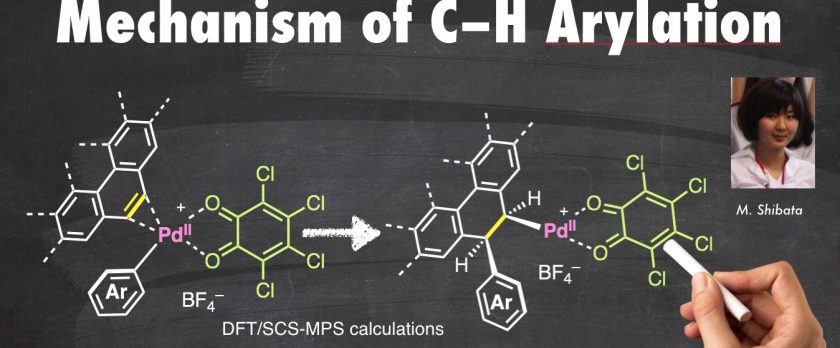
Tansition-metal-catalyzed C–H arylation of aromatic hydrocarbons represents a useful and ideal method for the production of biaryls and multiarylated aromatic compounds. We have previously reported the palladium-catalyzed direct C–H arylation of polycyclic aromatic hydrocarbons, such as phenanthrene, pyrene, and corannulene with various organosilicon, -borane, and -germanium compounds. In these reactions, o-chloranil proved to be an essential and unique promoter (stoichiometrically as an oxidant) and arylation occurred exclusively at the K-region. Herein, we report our mechanistic investigation of Pd/o-chloranil catalysis in C–H arylation of phenanthrene with trimethylphenylsilane by computational calculations. The results revealed that C–H arylation occurs through a sequence of transmetalation, carbometalation, and trans-β-hydrogen elimination steps. In addition, the triple role of o-chloranil as a ligand, oxidant, and base is also elucidated.
(For other applications of the present mechanistic investigation to homocoupling reaction of aryltrimethylsilanes by Pd/o-cholranil catalytic system, see: Mari Shibata, Hideto Ito,* Kenichiro Itami* Chem. Lett. 2017, 46, 1701–1704. DOI: 10.1246/cl.170723.)。