

Kenta Kato, Yasutomo Segawa, Lawrence T. Scott, and Kenichiro Itami
Angew. Chem. Int. Ed. 2018, DOI: 10.1002/anie.201711985
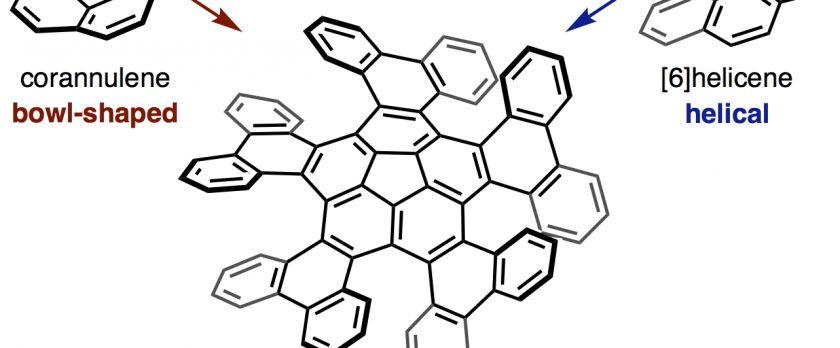
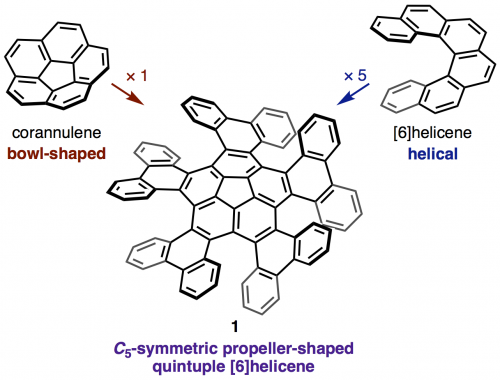 The synthesis and structural analysis of a quintuple [6]helicene with a corannulene core is reported. The compound was synthesized from corannulene in three steps including a five-fold intramolecular direct arylation. X-ray crystallographic analysis revealed a C5-symmetric propeller-shaped structure and one-dimensional alignment in the solid state. The enantiomers of the quintuple [6]helicene were successfully separated by HPLC, and the chirality of the two fractions was identified by CD spectroscopy. A kinetic study yielded a racemization barrier of 34.2 kcal/mol, which is slightly lower than that of pristine [6]helicene. DFT calculations indicate a rapid bowl-to-bowl inversion of the corannulene moiety and a step-by-step chiral inversion pathway for the five [6]helicene moieties.
The synthesis and structural analysis of a quintuple [6]helicene with a corannulene core is reported. The compound was synthesized from corannulene in three steps including a five-fold intramolecular direct arylation. X-ray crystallographic analysis revealed a C5-symmetric propeller-shaped structure and one-dimensional alignment in the solid state. The enantiomers of the quintuple [6]helicene were successfully separated by HPLC, and the chirality of the two fractions was identified by CD spectroscopy. A kinetic study yielded a racemization barrier of 34.2 kcal/mol, which is slightly lower than that of pristine [6]helicene. DFT calculations indicate a rapid bowl-to-bowl inversion of the corannulene moiety and a step-by-step chiral inversion pathway for the five [6]helicene moieties.